When the energy from photons is absorbed by matter the matter can emit electrons. The photoelectric effect refers to the emission or ejection of electrons from the surface of generally a metal in response to incident light.
Photoelectric Effect In Chemistry
Google Classroom Facebook Twitter.
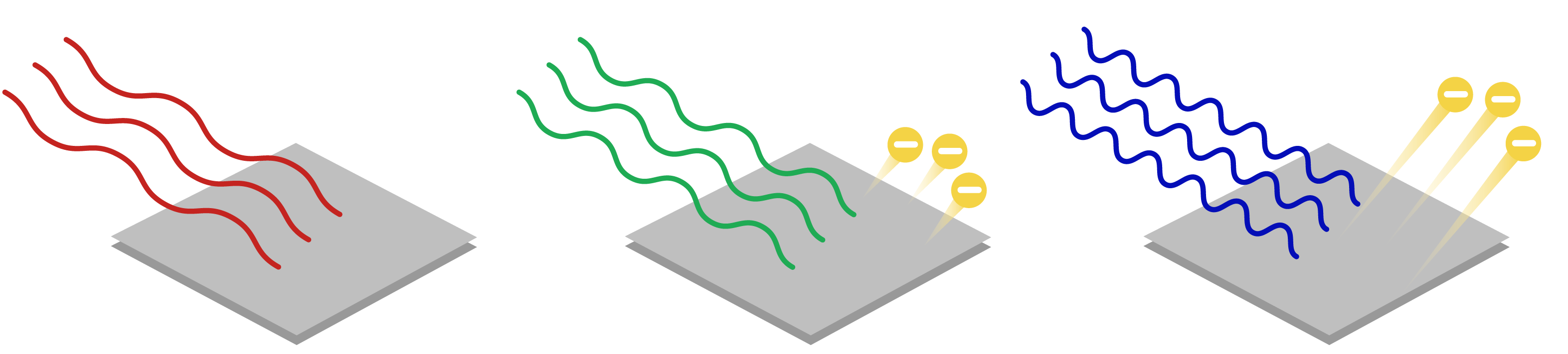
Photoelectric effect for dummies. Electromagnetic waves the electromagnetic spectrum and photons. How these experiments led to the idea of light behaving as a particle of energy called a photon. Explain the properties of an absorbed or emitted photon in relationship to an electronic transition in an atom or molecule.
The emitted electrons are called photoelectrons. The effect is often defined as the ejection of electrons from a metal plate when light falls on it. The Photoelectric Effect When ultraviolet light is shone on certain metal surfaces electrons are emitted.
The photovoltaic effect is the fancy name given to the phenomenon of converting light to electricity in a conventional solar panel. The effect is also called the Hertz Effect because it was discovered by Heinrich Rudolf Hertz but this name is not used often. Photoelectric effect phenomenon in which electrically charged particles are released from or within a material when it absorbs electromagnetic radiation.
Einstein and Millikan described the photoelectric effect using a formula in contemporary notation that relates the maximum kinetic energy Kmax of the photoelectrons to the frequency of the absorbed photons f and the threshold frequency f0 of the photoemissive surface. This process is called the photoelectric effect. There should be a delay on the order of seconds between the radiations contact with the metal and the initial release of photoelectrons.
This phenomenon whereby electrons in atoms get liberated by the absorption of energy from incident light is called the photoelectric effect. The electron configuration of the species. When electromagnetic radiation interacts with an atom it either excites electrons to a higher energy level known as an excited state or if the energy of the light is sufficiently high it can ionize the atom by removing the electron.
The interactions between the electrons and the nucleus. Photoelectric effect for dummies noviembre 13 2016 tuti Today in what I take my third coffee between rest and rest of the studies I want to tell you about the photoelectric effect since these days I have been talking about things of photons that are able to move things. By the end of this lesson students should be able to.
The effect is based on the idea that electromagnetic radiation is made of a series of particles called photons. Electricity is simply a flow of electrons running around a closed circuit. In the photoelectric effect electrons are emitted from matter typically metals and non-metallic solids as a consequence of their absorption of energy from electromagnetic radiation of high frequency short wavelength such as ultraviolet light.
At the high photon energies comparable to the electron rest energy of 511 keV Compton scattering may occur pair production may take place at energies over 1022 MeV. An illustration detailing the emission of photoelectrons as a result of the photoelectric effect is provided below. Bohrs model of the hydrogen atom.
When a photon hits an electron on a metal surface the electron can be emitted. The photoelectric effect is a property of light that is not explained by the theory that light is a wave. Kmax h f f0.
The photoelectric effect is a phenomenon in physics. This is one of the main reasons that scientists chose to treat light as both a wave and a stream of particles. So how do we create a flow of electrons using a solar PV panel and sunshine.
The photoelectric effect has helped physicists understand the quantum nature. Explaining the experiments on the photoelectric effect. The photoelectric effect should occur for any light regardless of frequency or wavelength.
The photoelectric effect is the observation that many metals emit electrons when light shines upon them. Energy contained within the incident light is absorbed by electrons within the metal giving the electrons sufficient energy to be knocked out of that is emitted from the surface of the metal. When you shine light onto metal as the figure above shows you get emitted electrons.
The electrons absorb the light you shine and if they get enough energy theyre able to break free of the metals surface. Special thanks to Kavinenh and Yi Hang for their contribution on making this video. What is the photoelectric effect.
The photoelectric effect occurs because the electrons at the surface of the metal tend to absorb energy from the incident light and use it to overcome the attractive forces that bind them to the metallic nuclei. Electrons emitted in this manner can be called photo. The photoelectric effect occurs with photons having energies from a few electronvolts to over 1 MeV.
Light with energy above a certain point can be used to knock electrons loose freeing them from a solid metal surface according to Scientific American.
 1 Photoelectric Effect 2 Photoelectric Effect What Is It When Metal Surfaces Are Exposed To Electromagnetic Radiation With Sufficient Energy They Absorb Ppt Download
1 Photoelectric Effect 2 Photoelectric Effect What Is It When Metal Surfaces Are Exposed To Electromagnetic Radiation With Sufficient Energy They Absorb Ppt Download
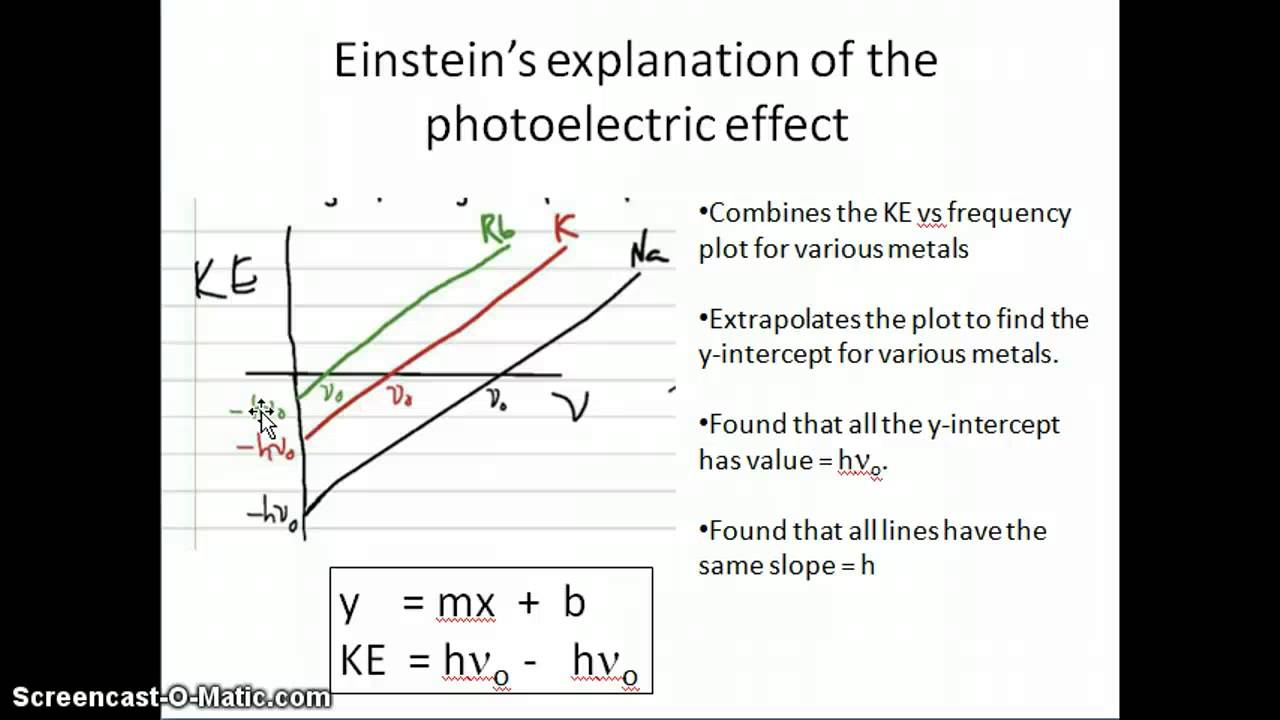 Einstein S Explanation Of The Photoelectric Effect Youtube
Einstein S Explanation Of The Photoelectric Effect Youtube
 Photoelectric Effect Wikipedia
Photoelectric Effect Wikipedia
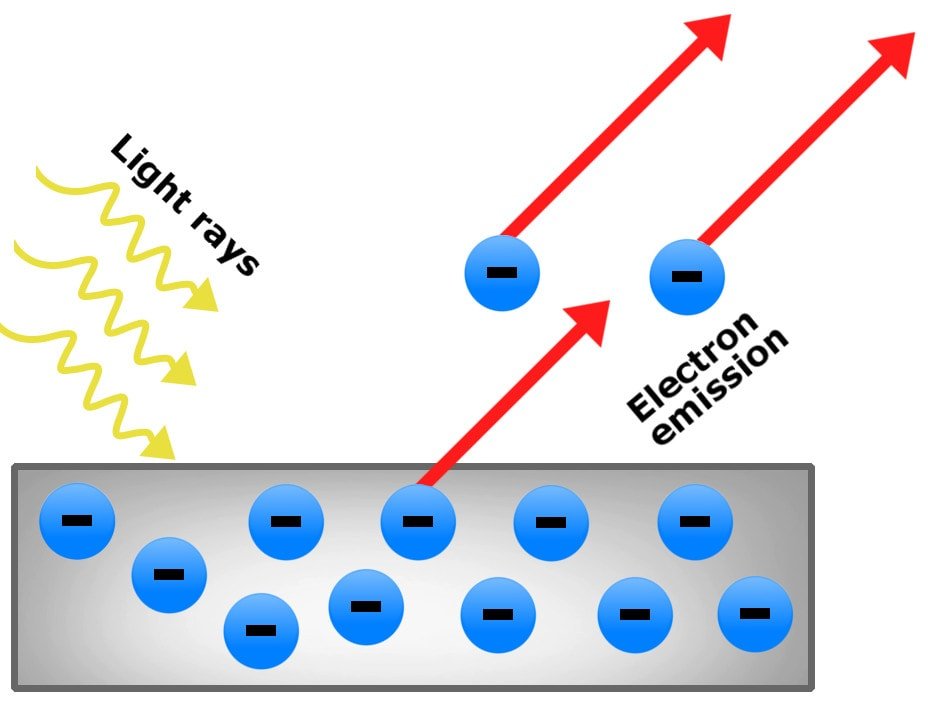 The Photoelectric Effect Atomstalk
The Photoelectric Effect Atomstalk
 Photoelectric Effect Article Khan Academy
Photoelectric Effect Article Khan Academy
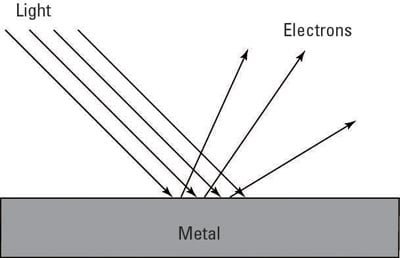 How Physicists Solved The Photoelectric Effect Of Light Dummies
How Physicists Solved The Photoelectric Effect Of Light Dummies
 Why Do Electrons Move Towards Anode In The Photoelectric Effect Experiment Physics Stack Exchange
Why Do Electrons Move Towards Anode In The Photoelectric Effect Experiment Physics Stack Exchange
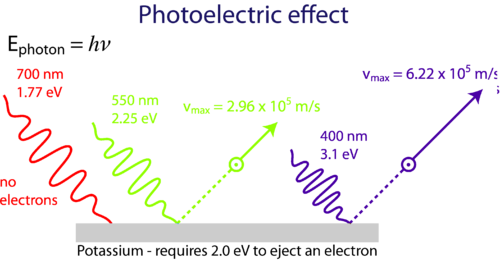 Photoelectric Effect Chemistry For Non Majors
Photoelectric Effect Chemistry For Non Majors
 Einstein S Explanation Of Photoelectric Effect Threshold Frequency Byju S
Einstein S Explanation Of Photoelectric Effect Threshold Frequency Byju S
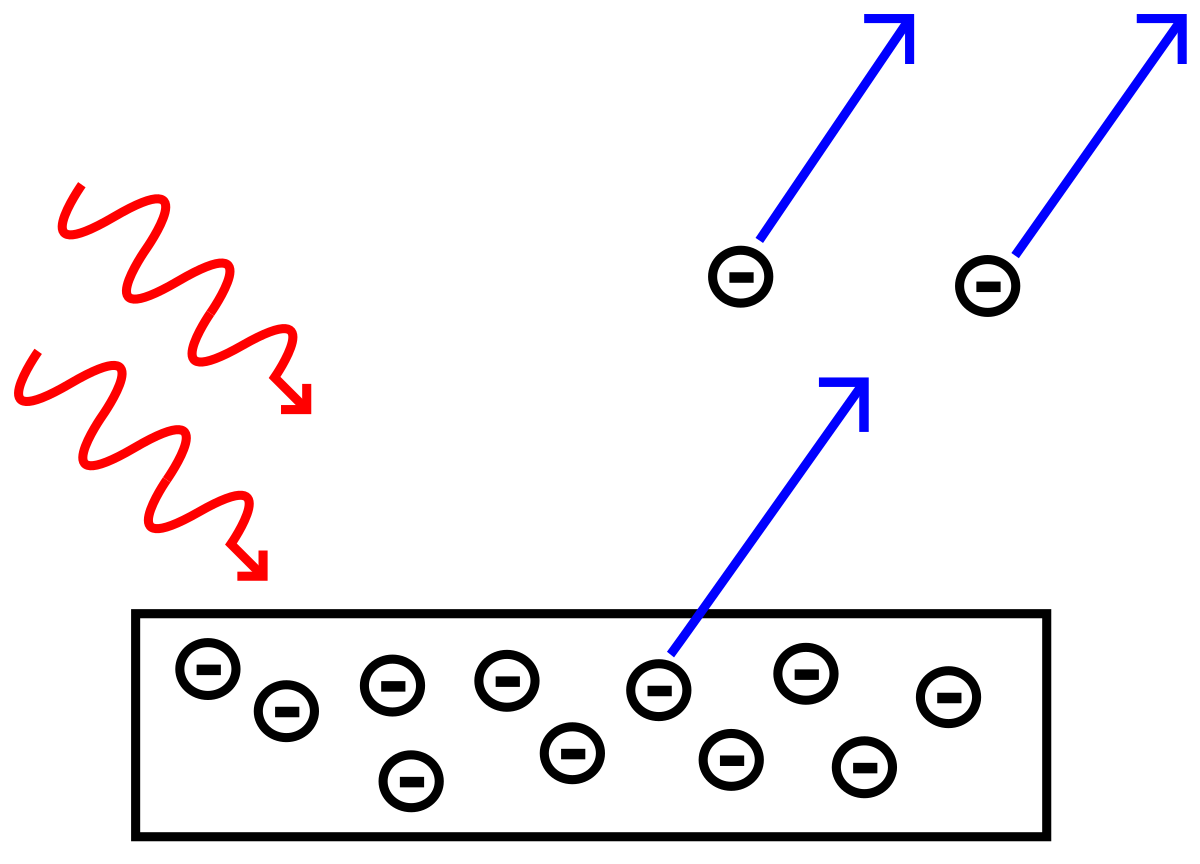 Photoelectric Effect Simple English Wikipedia The Free Encyclopedia
Photoelectric Effect Simple English Wikipedia The Free Encyclopedia
 Lecture 04 Outline Photoelectric Effect Experimental Facts Einstein S Explanation Problems Ppt Download
Lecture 04 Outline Photoelectric Effect Experimental Facts Einstein S Explanation Problems Ppt Download
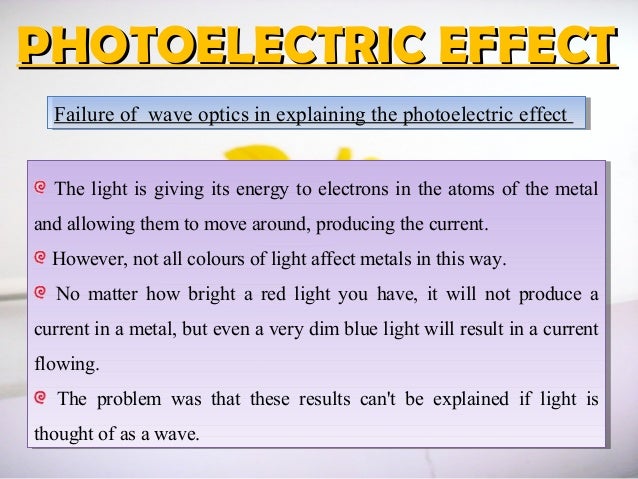 Chapter 3 Photoelectric Effect
Chapter 3 Photoelectric Effect
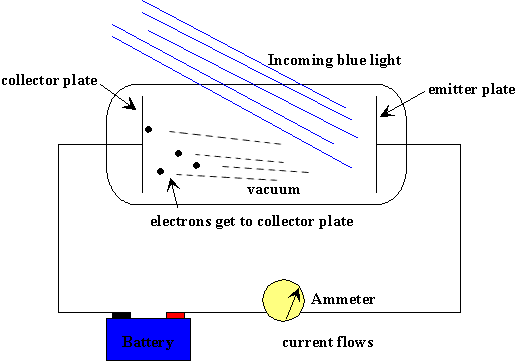 What Was Einstein S Explanation For The Photoelectric Effect Socratic
What Was Einstein S Explanation For The Photoelectric Effect Socratic
-
Digital Audio Output As their name implies the analog audio output transmits analog audio signalwhile the digital audio output transmits dig...
-
Past Participles are forms of verbs that express a completed action. Translate become in context with examples of use and definition. Past...
-
Weve tabbed out this guitar scales chart for you below. A Minor Scale Lessons - Scales. Pentatonic Minor Scale Guitar Patterns Chart Key O...
it's a good life pdf
It's a Good Life PDF Free Download . It's a Good Life. IT’S A GOOD LIFE By JEROME BIXBY Aunt Amy was out on the front porch, r...


